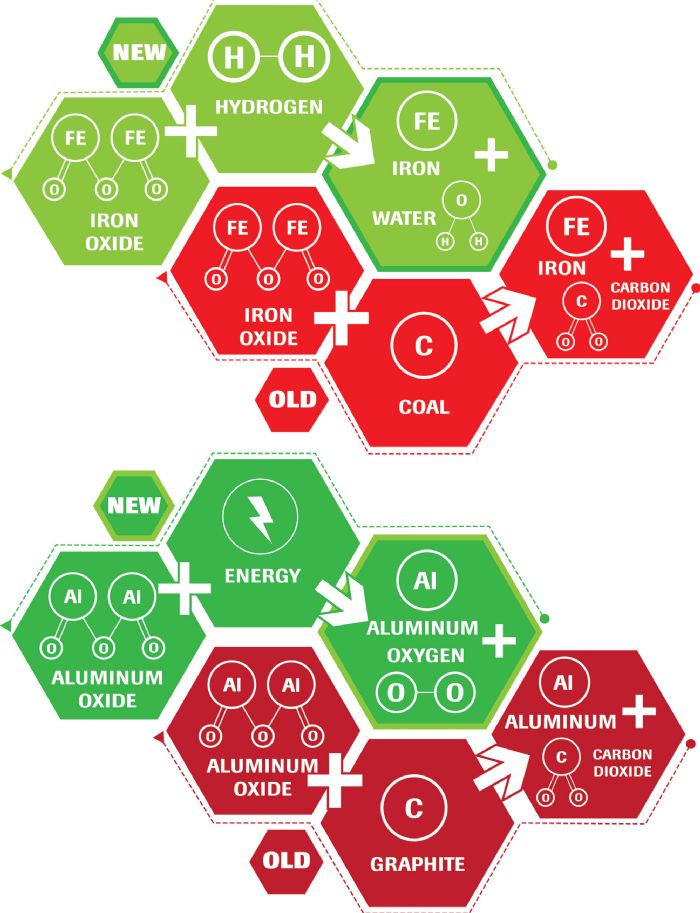
Breaking these bonds requires energy. A blast furnace combines carbon and iron oxide, providing the conditions for oxygen to preferentially combine with carbon rather than iron. This process yields liquid iron and carbon dioxide. The electrolytic process, commonly used to produce aluminum from aluminum oxide, uses consumable carbon anodes, with the reaction producing molten aluminum and carbon dioxide.
Green Aluminum Production
The electrolytic process commonly used to convert alumina to aluminum requires significant electrical energy. Emissions from power production account for more than 60 percent of the total emissions from aluminum production.
Historically, the industry relied on coal-fired power plants, but newer ones use renewable energy such as that produced by hydroelectric plants. The concentration of aluminum-production facilities around Lake Ontario and nearby waterways in part relates to access to hydroelectric power.
A new type of nonconsumable inert anode nearing commercialization negates the need for carbon anodes and emits oxygen as a byproduct of the reaction rather than carbon dioxide. Use of renewable-energy sources takes on heightened importance as this approach may require greater energy input.
Starting with recycled aluminum bypasses the significant energy input required to first convert bauxite to alumina and then reduce alumina to aluminum. Known as secondary aluminum, this is the most common pathway for aluminum production in the United States. The term secondary does not connote lower quality; its use distinguishes this route from the primary path starting with bauxite.
Remelting scrap comprised of different aluminum-alloy families results in a loss in properties, and an associated loss in value. While beverage-can aluminum has high recycling rates, this content is kept out of scrap used in automotive-aluminum production. Even the scrap from grades applied to automobile structural members will negatively impact the properties of skin panels, and vice versa. Scrap segregation, which avoids mixing different grades, is a critical strategy to retain scrap value.
Automakers increasingly employ closed-loop recycling strategies for cost savings as well as emissions reductions. Here, the stamping plant collects scrap aluminum from specific parts, all made from a particular grade ordered from a specific aluminum supplier, and returns the scrap to the supplier for remelting. Aluminum producers each have specific recipes for a given grade tailored to their equipment and manufacturing infrastructure, and this returned scrap flows seamlessly back into their process.
Key to scrap-based production: scrap availability. Consumers recycle less than half of all beverage cans, so the production of new cans obviously requires the higher-emissions primary aluminum to supplement the recycled content. Beverage cans have a relatively short turnaround time, unlike automotive aluminum where it may take several decades until that aluminum content becomes available for recycling. By 2035, some projections indicate that available automotive scrap will still meet less than 50 percent of demand.
Green Steel Production
Two production routes exist. One: integrated mills, which produce molten iron in a blast furnace (BF), followed by conversion of iron to steel in a basic oxygen furnace (BOF). An alternate approach is compact strip production (CSP), occurring in minimills that use an electric arc furnace (EAF) to melt primarily steel scrap. In the United States, the BF-BOF route represents 30 percent of production, with the remainder EAF-CSP. In the rest of the world, the percentages essentially are reversed due to the relatively lower availability of scrap for melting. Even with BF-BOF, the BOF charge uses approximately 25-percent scrap in addition to the iron coming from the BF.
Scrap-based steel production requires substantially less energy and emits less carbon dioxide than the integrated approach that starts with taconite. Although some minimills melt nearly 100-percent scrap, the products they make are better suited for items such as rebar and beams. Minimills producing sheet steels for stamped parts are more likely to add iron units to the melt, producing cleaner steels with improved properties. This iron could come from a blast furnace in the form of pig iron, or from products related to direct reduced iron (DRI).
Until recently, DRI production used either natural gas to create the H2 and CO gases required to reduce iron ore to iron, or coking coal as the carbon source for reduction. Although energy savings result as processing occurs below the melting temperature of iron, CO2 emissions are a byproduct of this approach.
New steel-production techniques rely on hydrogen instead of carbon to reduce iron oxide to iron. Green hydrogen is the term given to hydrogen produced from renewable-energy sources such as hydropower. DRI produced from green hydrogen results in minimal emissions, becoming one of the greatest potential decarbonization approaches for the steelmaking industry. Green hydrogen is not yet commercially viable, but industry analysts project that some mills will reach commercialization in coming years. Other approaches are under investigation as well, but none appear to have near-term commercialization potential at a comparable scale.
In general, the BF-BOF approach to steelmaking results in more emissions than the EAF-CSP approach, but U.S. blast-furnace emissions are substantially below the world average, with much of the gaseous emissions captured and used to provide heat and energy for additional chemical reactions. Other companies use the carbon-based emissions from steelmaking as feedstock in the production of products such as ethanol, as well as building materials. Carbon capture, use and storage efforts at many steelmakers are a piece of the puzzle to attain net-zero emissions.
U.S steel mills offer steel produced using sustainable practices such as renewable energy, and automakers have signed purchase agreements allowing them to offer a more environmentally friendly fleet. Some have even taken equity shares in green-steel production startups. Furthermore, the closed-loop recycling strategies described for aluminum scrap are expanding to sustainable steels, reinforcing the importance of the circular economy on an industrial scale.
Energy and Emissions Reductions from New Grades
Vehicle safety demands sheet metals of the highest strength. Stamping of these highest-strength parts relies on warm and hot forming of steel and aluminum alloys. Forming at elevated temperatures up to 900 C, requires the energy and emissions from the necessary furnaces. Steel and aluminum producers are creating formable cold-stamping alloys of nearly comparable strength to their counterparts formed at elevated temperatures to offer users additional options to create complex high-strength stampings. MF
See also: Engineering Quality Solutions, Inc., 4M Partners, LLC
Technologies: Materials
 Steel does not come out of the ground as steel; it exists as taconite, a combination of iron ore and other elements collectively described as impurities. Similarly, aluminum exists as bauxite in nature, a combination of alumina and impurities.
Steel does not come out of the ground as steel; it exists as taconite, a combination of iron ore and other elements collectively described as impurities. Similarly, aluminum exists as bauxite in nature, a combination of alumina and impurities.








